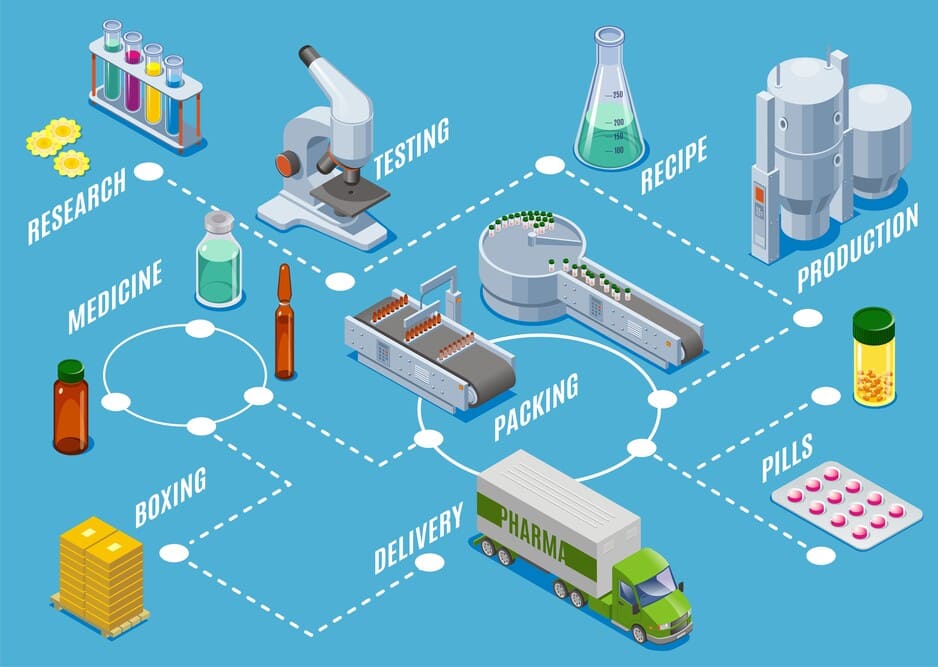
Optimizing Efficiency Through Process Flow
At Remedy Group, our manufacturing process is designed to deliver products of the highest quality and safety. We combine advanced technologies with strict adherence to global standards to ensure that every step of production, from raw material sourcing to final distribution, is carried out with precision and care.
Our process includes advanced techniques such as media and buffer preparation, seed cultivation, and production in bioreactors to ensure optimal yields. Steps like cell removal, filtration, and virus inactivation are implemented to guarantee product safety and purity.By combining innovation, expertise, and strict quality controls, we deliver pharmaceutical solutions that meet global safety and efficacy standards, supporting healthcare worldwide with reliability and excellence.
Manufacturing Process Flow
At Remedy Group, our manufacturing process combines advanced technology, precision, and strict compliance with global standards. Each step is carefully monitored to ensure the production of high-quality medicines and Active Pharmaceutical Ingredients (APIs). Here’s an overview of our comprehensive process
-
 Raw Material Sourcing
Raw Material SourcingWe source premium raw materials from trusted suppliers, ensuring purity and consistency. All materials undergo rigorous quality checks before entering production.
-
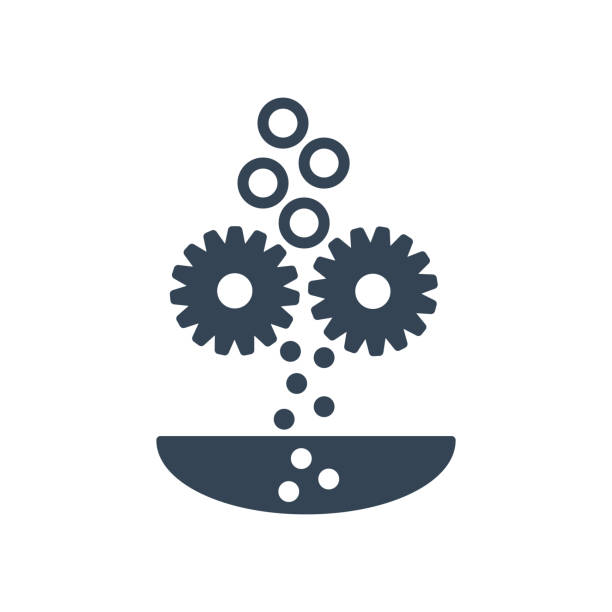 Media and Buffer Preparation
Media and Buffer PreparationPreparation of growth media and sterile buffers is performed under strictly controlled sterile conditions to create an optimal environment for critical production processes.
-
 Seed Cultivation
Seed CultivationHigh-quality seed cultures are developed to ensure consistency and precision during production. These cultures are expanded and prepared for scaling up in bioreactors.
-
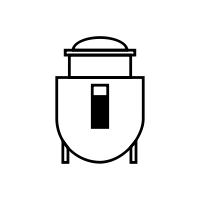 Production Bioreactor
Production BioreactorAdvanced bioreactors are used to produce the desired product under controlled conditions. This ensures optimal yield, purity, and quality of the final product.
-
 Cell Removal
Cell RemovalThe produced material is separated from cells using advanced filtration or centrifugation techniques to maintain the purity of the product.
-
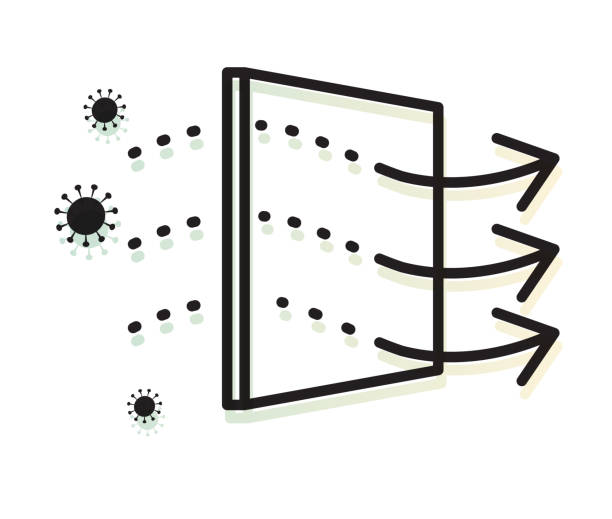 Ultra/Diafiltration
Ultra/DiafiltrationThe product undergoes ultra/diafiltration to concentrate and remove impurities, ensuring a high-purity intermediate ready for further processing.
-
 Virus Inactivation and Retention
Virus Inactivation and RetentionTo ensure safety, the product is subjected to virus inactivation and retention steps, removing any potential viral contaminants in compliance with global safety standards.
-
 Purification
PurificationAdvanced purification methods, including chromatography, are applied to isolate and refine the desired product, achieving high purity and potency.
-
 Packaging
PackagingThe final product undergoes rigorous testing, regulatory approval, and quality control checks before market distribution, ensuring safety, efficacy, and compliance with global standards.
-
 Final Quality Testing
Final Quality TestingEvery batch undergoes rigorous testing to verify compliance with safety, efficacy, and quality standards before being approved for release.
-
 Final Product Release and Approval
Final Product Release and ApprovalThe final product undergoes rigorous testing, comprehensive quality assurance, and stringent regulatory approval processes before market distribution.
-
 Distribution and Logistics
Distribution and LogisticsThe finished products are transported through a highly reliable and efficient supply chain to ensure timely and safe delivery to diverse markets worldwide.
This detailed and precise manufacturing process allows Remedy Pharma to produce safe, effective, and high-quality products, supporting global healthcare needs with excellence and reliability.


